Metal Usage Guide
Location and setting are key determining factors when selecting alloys as each alloy performs differently.
In urban or rural areas with low exposure to pollutants and salts, alloy 304, while often specified for interiors, can be an economical choice for an outdoor installation. When a high degree of corrosion resistance is required, alloys with a high content of chromium and molybdenum, like alloy 316 and alloy 2205, will perform best but also come at a premium price.
Refer to the Metal Usage Guide for more details including sources.
Stainless steel, like all types of steel, is not a single metal but an alloy, a material made from two or more separate elements alloyed or melted together. What all steels have in common is that their major ingredient or alloying element is the metal iron (Fe), to which a small amount of carbon (C) has been added Dictionary.com. ‘Alloy’ (2018)1International Stainless Steel Forum. ‘Introduction to Stainless Steel’ (2018)2. Stainless steel is an iron alloy that has a minimum chromium (Cr) content of 10.5% Cunat, Pierre-Jean. ‘Alloying Elements in Stainless Steel and Other ChromiumContaining Alloys’ (2018)3.
Characteristic of Stainless Steel
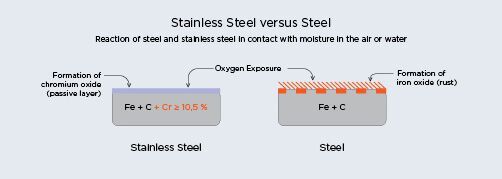
Stainless steel is often specified for its corrosion resistance, which is directly correlated to its chromium content. The chromium in stainless steel reacts with oxygen in the atmosphere to form a thin layer of chromium oxide, an insoluble film that is instantaneously formed on the surface. This natural chemical reaction forms a passive layer which acts to protect the material and has the ability to self-repair. Whereas steel, with no chromium content forms a layer of iron oxide, or rust, when exposed to air or water. See illustration below ArcelorMittal Paris. ‘Stainless Steel and Corrosion’ (2010)4.
Protective Passive Layer on Stainless Steel
Molybdenum (Mo) can be added to the alloy to further increase corrosion resistance. Grades of stainless steel are characterized by the composition of alloying elements. The higher percentage of chromium and molybdenum elements the higher the corrosion resistance of the alloy.
Selecting the wrong alloy for your project could lead to premature corrosion and even structural failure. Learn more about stainless steel categories and the breakdown of alloy composition and elements in the complete Metal Usage Guide(pdf).
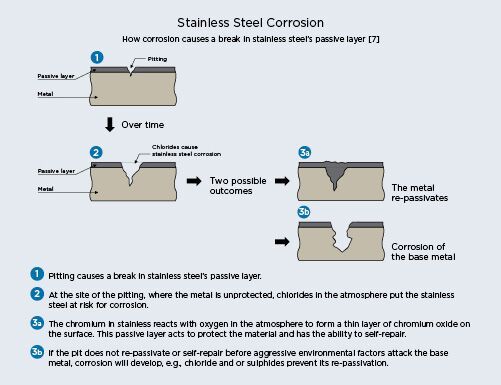
The naturally occurring passive layer of stainless steel may be attacked by the presence of corrosive material or if there is insufficient oxygen available to the surface disabling its selfrepairing nature. Pitting and crevice corrosion are the most common attacks on stainless steel.
Pitting and crevice corrosion
Pitting is usually characterized by very tiny dark brown pits on the surface. Crevice corrosion is essentially the same but on a larger scale and is usually caused by contact with a non-stainless steel material, like a washer, that creates a crevice on the surface. Pits or crevices form pockets in the surface of the material where oxygen cannot circulate adequately for the protective passive layer to reform and disrupts the micro structure of the metal.
Atmospheric substances, like air and water, and chlorides in airborne sea spray, rain, and dry salt particles carried by wind, may cause pitting and rusting of stainless steels. Earth’s natural environment of oxygen and condensed water vapor is itself sufficient to cause gradual corrosion of iron and steel surfaces through the creation of iron oxide unless a sufficiently corrosion resistant grade is chosen.
Corrosion alters the micro structure of the metal and its passive layer and drastically reduces the mechanical strength and useful life of the metal. This is why all stainless steel requires some level of maintenance depending on the alloy and where it is installed Specialty Steel Industry of North America. ‘The Care and Cleaning of Stainless Steel’6.
Understanding the environment and site conditions and then choosing the right alloy is critical to the success of your project.
Environmental and Atmospheric Conditions
The chlorides in rainwater are primarily marine salts carried inland by weather patterns. Generally, locations within 10 to 20 km of salt water are considered at risk for chloride-related corrosion, but local weather patterns and the performance of metals near the site should be evaluated prior to material selection. In some locations salt could be carried much further inland. The map below shows the average chloride concentration in rainfall across North America, milligrams per liter (mg/L) International Molybdenum Association. ‘Designer Handbook: Stainless Steel for Coastal and Salt Corrosion’ (2018)8.

Microclimates
In addition to known environmental factors, local site-specific microclimates and potential changes to the environment should be considered before a final stainless steel grade selection is made.
A microclimate is a local set of atmospheric conditions restricted to a very small area that differs from those in the surrounding area Dictionary.com. ‘Microclimate’ (2018)9. Areas particularly impacted by these microclimates are coastal locations or near chemical plant chimneys British Stainless Steel Association. ‘Selection of Stainless Steels for Building External Applications’ (2018)10, where unexpected acid condensates can form.
In contrast, factors such as low temperatures and low humidity reduce the risks of corrosion and can mean that a steel grade perhaps not thought suitable for a particular site may be worth considering.
Architectural sites are categorized as the following Nickel Institute. ‘Stainless Steels in Architecture, Building and Construction. Guidelines for Corrosion Prevention’ (2014)11:
| Site Type | Description |
|---|---|
| Urban | Residential, commercial or light industrial areas with low to moderate pollution from vehicular traffic and similar sources. |
| Industrial | Locations with moderate to heavy atmospheric pollution usually in the form of sulphur and nitrogen oxides, a bi-product of burning coal, oil, diesel and natural gas from power plants. |
| Rural and Suburban | Unpolluted, inland sites away from industrial atmospheric discharges or coastal or de-icing salts. Areas with low population densities and light, non-polluting industry may also be categorized as rural. |
| Marine/Deicing Salt | Areas where windborne sea spray and dry salt particles containing concentrated chlorides are present. |
After classifying a site, it is important to consider any changes that may occur during the design life of a proposed building or project. For example, will the environment get more polluted or cleaner over time? These considerations can have impactful implications and could potentially change the way in which a site should be classified.


Recommended alloy usage by architectural site and corrosion exposure.
| Site Type | Corrosion Exposure | Alloy 304 | Alloy 316 |
|---|---|---|---|
| Urban | Low | ||
| Medium | |||
| High | () | ||
| Industrial | Low | () | |
| Medium | () | ||
| High | () | ||
| Rural and Suburban | Low | ||
| Medium | |||
| High | |||
| Marine/Deicing Salt | Low | ||
| Medium | () | ||
| High | () |
DISCLAIMER
Information in this document is indicative only. Mechanical property requirements vary widely with temper, product and product dimensions. All information is based on our present knowledge and is given in good faith. No liability will be accepted by Geo. Bezdan Sales Ltd. (the Company) in respect of any action taken by any third party in reliance thereon.
The information provided has been drawn from various recognized sources, recognized industry references and manufacturer’s data. See Appendix B: References. No guarantee is given that the information is from the latest issue of those sources or about the accuracy of those sources.
Material supplied by the Company may vary significantly from source data, but will conform to all relevant and applicable standards. As the products detailed may be used for a wide variety of purposes and as the Company has no control over their use, the Company specifically excludes all conditions or warranties expressed or implied by statue or otherwise as to dimensions, properties and/or fitness for any particular purpose, whether expressed or implied.
Advice given by the Company to any third party is given for that party’s assistance only and without liability on the part of the Company
CORROSION EXPOSURE
| Low | Least corrosive within that category due to low humidity and low temperatures |
| Medium | Fairly typical of category |
| High | Corrosion is likely to be higher than typical for the category due to persistent high humidity, high ambient temperatures, and/or particularly aggressive air pollution |
RECOMMENDED USE
| Most economical choice | |
| () | Indicates that the grade may be suitable if a smooth surface finish is selected and it is washed regularly |
| Good service, but may be overspecified | |
| Corrosion likely |


| Alloy | Usage | Common Applications | |
|---|---|---|---|
 | Alloy 201 | Ideal for indoor architectural applications | Architectural Applications: Indoor and non-structural architecture. |
| Not ideal for outdoor use | Other Applications: Cutlery and cookware, food and beverage equipment, automobile parts. | ||
 | Alloy 304 | Ideal for all indoor architectural applications Ideal for outdoor use in rural and urban environments with typical atmospheric conditions, i.e., moderate exposure to humidity, temperature and pollution Option for indoor or outdoor use in industrial and marine environments with typical atmospheric conditions if precautions are taken for specified cleaning and maintenance | Architectural Applications: Indoor and non-structural architecture. |
| Not ideal for indoor or outdoor use in industrial and marine environments with exposure to harsh atmospheric conditions (i.e. high humidity, high temperature and high levels of pollution) | Other Applications: Sinks, pharmaceutical equipment, wheel covers, truck bodies and exhaust manifolds. | ||
 | Alloy 316 | Ideal for indoor and outdoor use in industrial and marine environments with typical atmospheric conditions Option for indoor or outdoor use in industrial and marine environments with typical atmospheric conditions if precautions are taken for specified cleaning and maintenance | Architectural Applications: General architecture. |
| Not required for general indoor architectural applications. Often over specified for outdoor use in rural and urban environments with moderate to harsh atmospheric conditions, i.e., moderate to harsh exposure to humidity, temperature and pollution | Other Applications: Chemical storage tanks, pressure vessels and piping. | ||
 | Alloy 2205 | Ideal for indoor and outdoor use where in close proximity to swimming pools or highly chlorinated environments Ideal for indoor and outdoor use in Industrial and Marine environments with harsh atmospheric conditions | Architectural Applications: Pool enclosures or railings. |
| Not required for general indoor architectural applications. Often over specified for outdoor use in rural and urban environments with moderate to harsh atmospheric conditions, i.e., moderate to harsh exposure to humidity, temperature and pollution | Other Applications: Stainless structures submerged in chlorinated solutions, hot water boilers and brewing tanks. | ||
Download a copy of the complete Metal Usage Guide for easy reference and additional information including:
Stainless Steel Alloy Elements
| Element | Properties |
|---|---|
| Carbon (C) |
|
| Chromium (Cr) |
|
| Iron (Fe) |
|
| Molybdenum (Mb) |
|
| Manganese (Mn) |
|
| Phosphorus (P) |
|
| Sulfur (S) |
|
| Silicon (Si) |
|
| Nickel (Ni) |
|
| Nitrogen (N) |
|
Stainless Steel Alloy Classification
Ferritic Steel Alloy 409 |
|
Austenitic Steel Alloy 201, 304 and 316 |
|
Martensitic Steel Alloy 410 |
|
Duplex Steel Alloy 2205 |
|
| ALLOY | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Alloy Components | Alloy Properties | ||||||||
| Chromium (Cr) | Molybdenum (Mb) | Nickel (Ni) | Corrosion Resistance | Strength | Suitable for Architecture | Magnetic | Cost | ||
| STEEL | Ferritic Steel Alloy 409 | Low | None | Low | Reduced | Moderate | No | Yes | Low |
Austenitic Steel Alloy 201, 304 and 316 | Higher | Higher | High | Relative to Alloy* | Relative to Alloy* | Yes | No | Relative to Alloy* | |
Martensitic Steel Alloy 410 | Higher | Higher | Low | Reduced | High | No | Yes | Low | |
Duplex Steel Alloy 2205 | High | Low | Low | High | High | Yes | Yes | High | |
* Corrosion resistance is relative to the alloy.
e.g., Alloy 201 is austenitic but has low corrosion resistance.
Stainless Steel Alloy Composition
| STEEL TYPE | ||||||
|---|---|---|---|---|---|---|
Ferritic Steel Alloy 409 | Austenitic Steel AISI 201* | Austenitic Steel AISI 304* | Austenitic Steel AISI 316* | Duplex Steel AISI 2205* | ||
| ELEMENT (%) | Carbon (C) | 0.01% | 0.15% maximum | 0.08% maximum | 0.08% maximum | 0.03% maximum |
| Chromium (Cr) | 11% | 16% - 18% | 18% - 20% | 18% maximum | 22% - 23% maximum | |
| Iron (Fe) | Balance | Balance | 66.345% - 74% | 62% | < 0.3% | |
| Molybdenum (Mb) | — | — | — | 3.0% maximum | 3.0% - 3.5% | |
| Manganese (Mn) | 0.30% | 5.5% - 7.5% | 2.0% maximum | 2.0% maximum | 2.0% maximum | |
| Nickel (Ni) | 0.25% | 3.5% - 5.5% | 8% - 10.5% | 10% - 14% | 4.5% - 6.5% maximum | |
| Nitrogen (N) | 0.012% | 0.25% maximum | 0.10% maximum | 0.10% maximum | 0.08% - 0.20% | |
| Phosphorus (P) | 0.023% | 0.03% maximum | 0.045% maximum | 0.045% maximum | 0.020% maximum | |
| Silicon (Si) | 0.41% | 1.0% maximum | 1.0% maximum | 1.0% maximum | 1.0% maximum | |
| Sulphur (S) | 0.001% | 0.06% maximum | 0.03% maximum | 0.03% maximum | 0.03% maximum | |
* Bezdan choice of metals. Steel is classified as per the American Iron and Steel Institute (AISI) standardize numbering system for steels
References
| [1] | Dictionary.com. ‘Alloy’ (2018), |
| [2] | International Stainless Steel Forum. ‘Introduction to Stainless Steel’ (2018), |
| [3] | Cunat, Pierre-Jean. ‘Alloying Elements in Stainless Steel and Other Chromium-Containing Alloys’ (2018), |
| [4] | ArcelorMittal Paris. ‘Stainless Steel and Corrosion’ (2010), online: https://silo.tips/download/stainless-steel-and-corrosion |
| [5] | American Welding Society. ‘Classifications of Stainless Steel’ (2018), online: app.aws.org/wj/1998/11/kotecki/ |
| [6] | Specialty Steel Industry of North America. ‘The Care and Cleaning of Stainless Steel’, |
| [7] | ArcelorMittal Paris. ‘Stainless Steel and Corrosion’ (2010), online: www.aperam.com/uploads/stainlesseurope/Brochures/Leaflet%20corrosion_Eng_374Ko.pdf |
| [8] | International Molybdenum Association. ‘Designer Handbook: Stainless Steel for Coastal and Salt Corrosion’ (2018), online: www.nemaenclosures.com/blog/wp-content/uploads/2012/09/stainless-steel-corrosion.pdf |
| [9] | Dictionary.com. ‘Microclimate’ (2018), |
| [10] | British Stainless Steel Association. ‘Selection of Stainless Steels for Building External Applications’ (2018), |
| [11] | Nickel Institute. ‘Stainless Steels in Architecture, Building and Construction. Guidelines for Corrosion Prevention’ (2014), |
| [12] | Ispat Digest. ‘Phosphorus in Steels’ (2018), |
| [13] | The Balance. ‘Metal Profile: Iron’ (2018), |
| [14] | British Stainless Steel Association. ‘Magnetic Properties of Ferritic, Martensitic and Duplex Stainless Steels’ (2018), |
| [15] | Greenwood Magnetics. ‘Stainless Steel: The Magnetic Properties of 304 and 316 Stainless Steel’ (2018), online: greenwoodmagnetics.com/resource/what-is-the-difference-between-304-and-316-stainless-steel/ |
| [16] | Woodford, Chris. ‘Iron and Steel’ (2018), |
| [17] | Bodycot. ‘S3P – Sulfur in Corrosion Resistant Steels’ (2018), |

Maintain Stainless Steel Performance and Life
Reduce the risk of corrosion from pollutants, salts and chlorides with regular stainless steel care and maintenance.